PFAS Destruction
Only the most aggressive and energy-intensive technologies are capable of breaking down PFAS. Therefore, cost-efficient treatment options for large volumes of contaminated water rely on coupling technologies that first separate and concentrate PFAS to reduce the volumes and make destructive treatment technologies viable.
Today, destruction technologies like electrochemical oxidation (ECO) and plasma have demonstrated abilities to breakdown the stubborn carbon-fluorine bonds, the strongest in chemistry, at the heart of all PFAS. Recirculating PFAS in the environment from one media to another draws significant concern worldwide, as we risk re-releasing it back into our environment. Therefore, destruction is a critical step in solving the global PFAS crisis. To make destruction feasible, though, the entire treatment train must be optimized for maximum efficiency.
Separate and Concentrate
In addition to conventional methods like GAC and AIX to reverse osmosis and beyond, our researchers have been rigorously testing new ways to separate and concentrate PFAS molecules, exploiting their affinity for air-water interfaces. One of these technologies relies on using air bubbles to “strip” PFAS out of water and into foams, which are condensed to generate hyper-concentrated PFAS solutions.
Concentration is a critical step in the destruction treatment train because it drastically reduces the volume of PFAS-laden effluent on its way to destruction. Together with EPOC Enviro, CDM Smith has been rigorously testing surface-active foam fractionation (SAFF®), which has been demonstrated to concentrate PFAS molecules up to 42,000 times with ongoing development to achieve concentration factors closer to one million times.
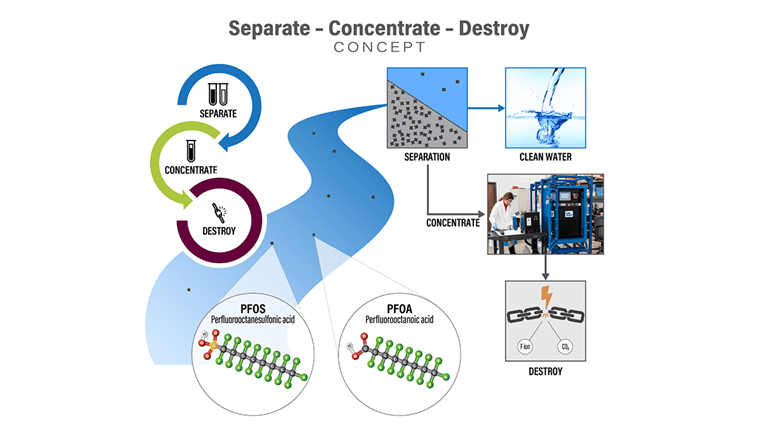
Destroy
There are numerous PFAS destruction technologies under development, conveniently summarized in this ITRC PFAS Fact Sheet. Promising ex-situ destruction technologies that have progressed from bench- to pilot-scale include electrochemical oxidation, plasma and UV reductive, hydrothermal, and supercritical water oxidation. These approaches have successfully degraded an array of high-concentration PFAS at the laboratory scale. However, not all are sufficiently mature yet to assess PFAS treatment costs and overall effectiveness with confidence at the field scale.
Because they are energy intensive, it is important to selectively employ currently available destruction technologies on highly-concentrated, low-volume targets. These include:
- AFFF concentrates
- Groundwater within PFAS source areas
- Remediation waste streams (such as wastewater generated from regeneration of GAC or regenerable ion exchange resin, foam fractionation-, soil washing-, rejected reverse osmosis- concentrates, chemical or electro-coagulation)
- Landfill or biosolid leachate
Electrochemical Oxidation (ECO)
Our researchers have proven electrochemical oxidation (ECO) to reduce high-concentration PFAS effectively, typically achieving reductions of 90-99.999% in laboratory studies.
ECO uses an electrochemical cell to generate an electric current between a reactive anode and cathode (the electrodes). The process degrades PFAS through two mechanisms:
- Anodic oxidation (direct electrolysis) – PFAS adsorb onto an anode surface and are destroyed directly at the electrode by a direct electron transfer reaction
- Indirect oxidation – Strong oxidizing and nonselective radicals (such as hydroxyl, oxygen, sulfate and carbonate) are generated in-situ that react with, and break down, PFAS in the bulk liquid reactions.
Choosing a Destruction Approach
Because of the emerging market for destructive PFAS technologies, they are often promoted hastily without demonstrating complete defluorination and without confidence the technology can meet stringent effluent discharge requirements. The feasibility of these technologies must be carefully considered for each new application. To effectively develop a treatment train approach, technology compatibility, engineering constraints, and operations and maintenance requirements must be considered. A thoughtful approach will ensure the system can meet required treatment volumes, rates, and discharge criteria.
Most destructive technologies for PFASs only work in specific conditions including extreme temperature or pressure, under caustic conditions, require high energy consumption, chemical additives, and other harsh treatment conditions.
The development and commercialization of PFAS destruction technologies will not be easy. Careful consideration and understanding of PFAS transformation and defluorination must be incorporated into the technology evaluation for a particular site/application with thoughtful design of bench and pilot scale systems to demonstrate technology(ies) and incorporate economic feasibility in the selection process.
Consideration of balanced technology benefits and limitations that should be discussed with technology providers include:
- High energy demand and feasibility of high energy/cost at the scale required for the system
- Health and safety concerns
- Feasibility of operating large-scale systems, if required
- Incomplete PFAS destruction resulting in accumulation of fluorinated intermediates that are generated but not measurable
- Feasibility of achieving stringent (i.e. very low) treatment requirements, often a treatment train approach may be needed before effluent discharge
- Effectiveness in destroying all PFAS chemicals, including short chain PFAS, which are generally harder to treat and precursors as sources of PFAAs
- Generation of non-PFAS toxic byproducts. For instance, perchlorate is known to be formed during electrochemical oxidation treatment due to the aggressive oxidation of chloride in the feedwater. Although perchlorate can be addressed easily, treatment systems must account for, and treat, perchlorate in the process
CDM Smith’s approach to assessing PFAS destructive technologies at a site includes treatability testing at the bench-, pilot-, and full-scale levels, using three lines of evidence to confirm complete PFAS destruction. Our collaboration with universities and research foundations allows us to explore the latest analytical methods for understanding the destructive mechanisms, kinetics, toxic intermediates, and end products formation. The collaboration helps bring the most effective means and methods to our projects. We are working on advancing the science and engineering into comprehensive treatment systems (e.g., treatment trains) to achieve a complete solution for PFAS-impacted waters.

Our approach has the potential to leverage synergistic technologies to provide a more sustainable solution for PFAS treatment.








